
- Due to increased concerns about pharmacovigilance, PV has become more crucial in the pharmaceutical industry.
- PV, according to the World Health Organization, is the science and methods involved in the ongoing detection, evaluation, and comprehension of adverse events or adverse medication reactions to determine the risk profile of a product.
- A primary national regulatory authority and numerous regional or national centers make up the common structure for a national pharmacovigilance system.
- According to WHO, National Centers are pharmacovigilance facilities authorized by the organization that are in nations taking part in the WHO Programme for International Drug Monitoring.
- Typically, NCs are affiliated with or a component of the national drug regulatory body. Individual case safety reports are sent to a local PV center by healthcare professionals and patients.
- The drug safety sector is currently under pressure from expanding data quantities and complexity to find solutions that lower case processing costs while maintaining compliance with globally changing standards and preserving or even improving the information quality in ICSRs
- In parallel to this, regulatory organizations are pressuring doctors to record more instances of PV, and patients are also sharing their accounts of adverse events.
- This entails making use of outsourcing partners’ advantages, which help manage workload demands and limit workforce expansion while delivering scalability. Although the opportunity for outsourcing is limited, the processing still requires manual labor.
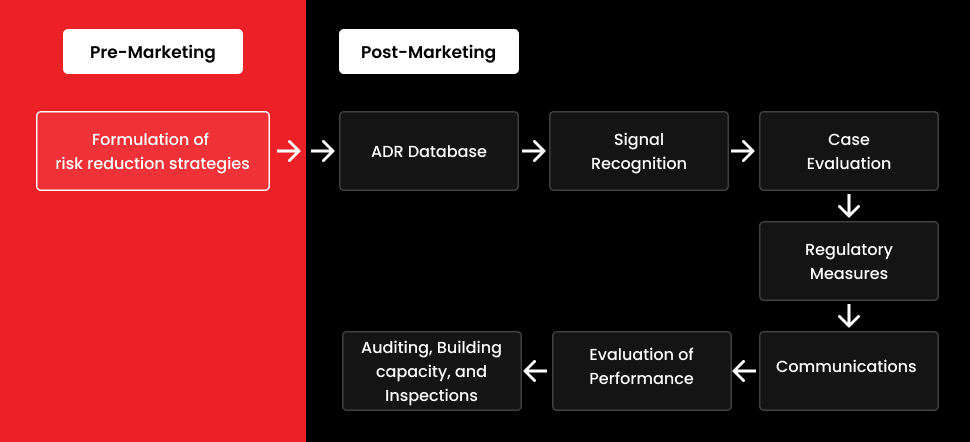
- Pharmacovigilance is a post-marketing tool that guarantees a drug’s safety. The detection of ADRs and the mitigation of related hazards are its main concerns. Regarding tiers of the social healthcare setting, a well-structured PV system can assist in producing correct safety data.
- A well-thought-out plan that guarantees flawless execution and tangible benefits is required for the establishment of a pharmacovigilance system.
Current Pharmacovigilance Challenges
PV systems have come a long way over the past few decades, but they still face several obstacles today. A well-designed PV system can assist in accurately generating safety data for several spheres of the social healthcare setting. The establishment of a pharmacovigilance system necessitates the harmonization of several criteria and a carefully thought-out strategy that guarantees flawless execution and physical benefits.
Situation 1: Inconsistent reporting of adverse events
- Adverse events, however, do not always occur while visiting the healthcare center. It can happen several hours after the medicine was administered. Patients frequently struggle to appropriately report AEs since they can’t recall every detail about them.
- When a patient disregards medical advice or experiences negative effects from drugs taken simultaneously with prescribed therapies, this is referred to as an adverse event. Such inaccurate reporting may cause drug safety committees to draw erroneous conclusions, which may result in the suspension or removal of medications.
Situation 2: Constantly changing laws and business practices
- PV systems must scale easily and effectively because of business expansion into newer markets. It has become crucial to ensure that PV systems and procedures continue to advance.
- The underlying database, configurability, reporting capacity, and system connection with data sources and other applications are just a few areas where the evolving regulations have an impact on PV operations.
- Regulation non-compliance and the resulting fines are caused by a lack of support and continually evolving standards. The need varies more widely in non-ICH regions, making reporting even more challenging.
Situation 3: Data Processing and Detection
- Because of the steadily increasing amounts of AE data, it is difficult for reviewers to track, identify, and manage all potential developing patterns using only qualitative methods. Since manual processes take a long time, it can take the reviewer a few weeks to study a particular signal.
- On the other hand, the regulators’ and life science enterprises’ query and response cycles are rapidly closing, forcing the reviewers to quickly assess the signals.
- Reviewers must concentrate on current problems while spending less time and effort identifying false signals. Ineffective signal detection and handling make it difficult to meet regulatory reporting requirements, which results in fines.
Situation 4: Operational efficiency and productivity
- When transferring and submitting cases across ICH areas, an organization faces many challenges. Some businesses choose to send the submission data back to their main office to keep the central system up-to-date.
- Routing and maintaining track of ICSRs must be done manually because pharmacovigilance is not automated. The procedure is delayed as a result, and AE processing and reporting are inadequate.
Situation 5: System Implementation
- Existing PV systems have problems with system integration, data sharing between unrelated applications, system availability, and system scalability. It leads to a system breakdown because of poor scaling and shaky performance.
- Data inconsistency results from the manual intervention involved in this. As a result, productivity and efficiency suffer in PV departments.
Situation 6: Handling a larger volume of data
- v Processing the growing amount of data that the ecosystem is producing is posing a serious issue for the worldwide PV business.
- v An annual exponential increase in data quantities is being caused by a variety of sources, including journals, publications, social media, patents, and an increasing number of unstandardized data sources.
- v However, many businesses continue to manage information using outdated technological platforms and manual procedures. This reduces productivity and is prone to mistakes.
Situation 7: Ensuring Data Quality
- The complexity and variety of the technologies used to gather and store the data are growing along with the volume and type of data being collected during the life cycle of the product.
- Certain data input and data coding standards are essential for accurate reports and signal detection.
- To maintain quality, newly entered or received data must be reviewed for quality.
- A PV system should adhere to certain specific parameters to reduce errors in signal detection, recorded data, and aggregate reporting.
Join Sollers College today to start your career in drug safety and pharmacovigilance. Your PV skills will be helped at every stage of the curriculum. Students who are ready to develop their profiles can choose from training programs offered by Sollers.
Sollers’ College distinctive curriculum consistently creates a wide range of career options and offers the best professional supervision and swift learning support.
To learn and share your knowledge, Sollers College created a road to the sizable pharmaceutical sector. Don’t let yourself be limited to meeting your needs in this pharmaceutical market.



