The Pharmacy profession has experienced significant change over the last two to three decades. Pharmacists can use Pharmacovigilance systems interfaced with electronic health records to observe the drugs they fill and recognize adverse drug reactions quicker than non-pharmacists, thereby decreasing expensive healthcare costs.
Pharmacovigilance information systems managed by pharmacists can recognize adverse drug reactions in emerging countries where quality control of medicines is questionable. Reports suggested that patients had insufficient knowledge about their prescribed drugs, although they had been using them for a while.
73% of pharmacists work in hospital or pharmacy settings, where they can face events based on adverse drug reactions or other drug-related problems. Their involvement in pharmacovigilance systems is crucial.
The development of electronic information systems has been a milestone in identifying and intervening drug-related problems such as dosage, adverse reactions, interactions, compliance, or ineffectiveness. Such decision support systems in electronic medical records can capture drug-drug interactions or identify other issues (e.g., contraindications) with prescriptions before they are filled
Pharmacists have a crucial role in the US and health systems to maintain the rational and safe use of medication, for they are drug experts who are specifically trained in this field. They ensure that the drugs in the marketplace we consume are generally safe, and all those identified as hazardous are taken off the market.
Pharmacovigilance is an excellent employment option for medical, pharmacy, and life science graduates. It has resulted in pharmacists taking on greater responsibility in managing minor illnesses and delivering public health interventions. There is no limit to the patients dealt with by pharmacists.
In the pharmaceutical sector, the number of career opportunities is nearly limitless. Pharmacists can play a role helping patients with chronic diseases have better medication at the correct time and get good clinical outcomes. Pharmacists serve as patient advocates, contributing information that permits patients to evaluate risk and improve their autonomy
The pharmaceutical industry in the US is one of the most advanced industries of the country and the third-largest in the world. It is technologically strong and self-reliant. Effective use of pharmacists’ workforce will improve the outcome of pharmacotherapy and decrease global health costs.
Pharmacists can fully use their clinical abilities and usually involve diagnosis and therapeutic management. More than ever before, community pharmacists are now in a position to identify, record, and report medication safety incidents.
Sollers’ Drug Safety and Pharmacovigilance certification help Pharmacists to enrich their career growth in the Pharmaceutical sector. It might seem like a lateral move at first, but it will open more fantastic opportunities in the long term.







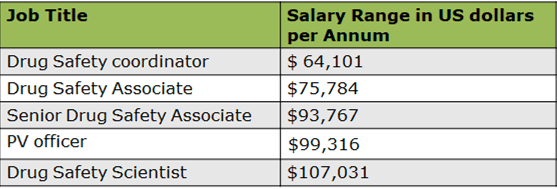
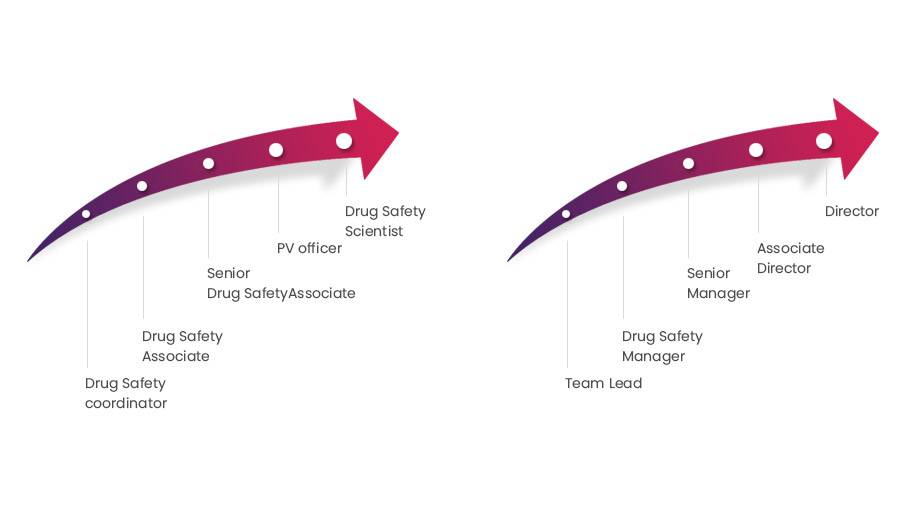 Drug Safety Coordinator:
Drug Safety Coordinator: