Guidelines for Literature Monitoring in Pharmacovigilance:
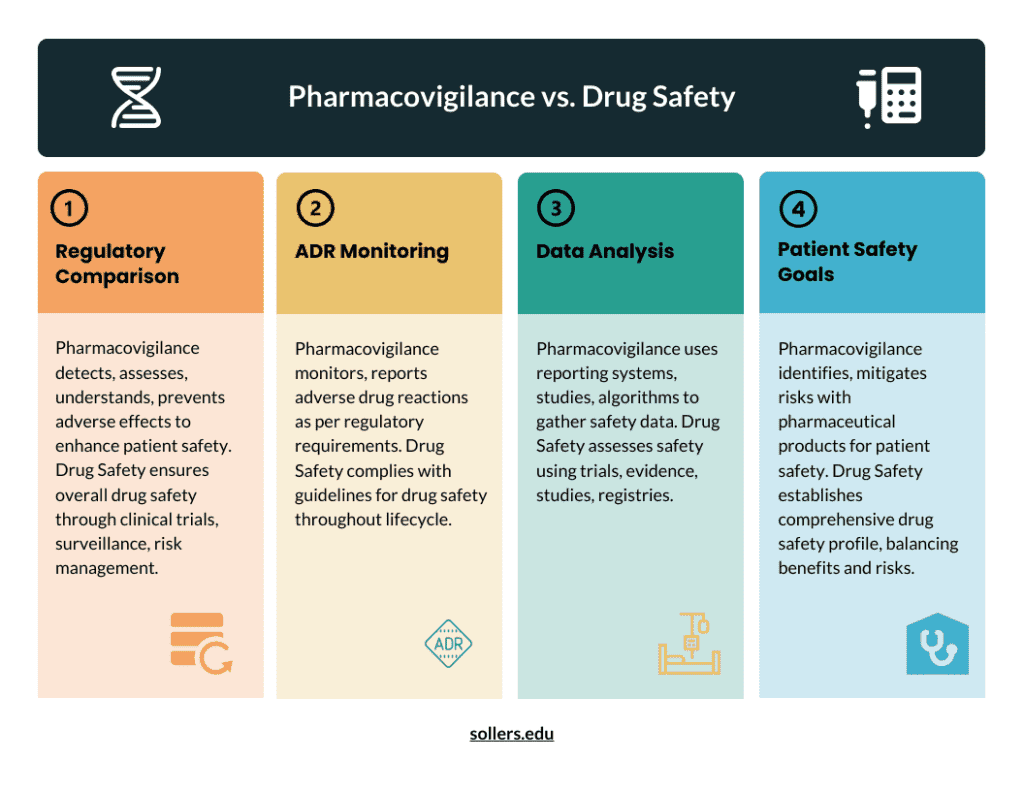
- Pharmacovigilance’s primary goal is to promote medical product security and efficiency. As soon as possible, information about the safety of these ingredients is made available to patients, medical professionals, and the wider community.
- Examining the creation, administration, and distribution of pharmaceuticals is part of pharmacovigilance. It is most likely the pharmaceutical market area most strictly regulated.
- ADRs involving pharmaceutical products are to be recognized, detected, evaluated, and reported through pharmacovigilance.
- Systematic tracking and assessment of medical literature is one of these requirements. In addition, a thorough search of medical journals for increasingly common adverse drug reactions.
- A manufacturer must have an effective pharmacovigilance system because flaws may compromise patient safety.
- Articles published, articles and reviews published in indexed or unindexed journals, content published online, posters, conference abstracts, etc.
- Literature monitoring covers all of them. A more in-depth analysis of regulatory reporting, signal detection, and aggregate reporting is carried out using regulatory reports, clinical trial reports, literature reports, and license partner reports.
- A useful tool for creating risk assessments is the individual safety report (ICSR). Marketing authorization holders regularly check popular reference databases to stay informed about upcoming publications.
According to regulatory guidelines for handling and reporting adverse events, adverse events that satisfy the ICSR requirements are handled. When relevant articles are found, they will be further screened to ensure that they meet four requirements:
1) identified source.
2) company product.
3) patient; and
4) adverse event.
Science and medicine publications should be used to support any analysis of a product’s safety profile. Whenever an emerging safety signal or safety concern appears, literature searches and monitoring are used to locate isolated reports of negative effects.
A Summary of the Latest Literature Monitoring Techniques
Processes can have unintended consequences when their foundation is compromised. Therefore, unbiased searching is essential for accurately and effectively monitoring medical literature.
Getting the most accurate results without adding extra data is more significant than ever due to the growing data volume. Literature monitoring involves two significant obstacles. The search strategy is the first challenge, and duplication is the second.
Designing the most appropriate search strategy and choosing the right databases
Marketing authorization holders must conduct clinical literature surveillance by GVP Module VI and based on the necessary frequency specified by the local regulatory authorities. This is for both locally (non-indexed) literature journals and globally indexed literature databases.
ICSRs, aggregate reports, and potential security data should all be considered when developing search strategies. To reduce the chance of missing pertinent ADR information, it is crucial to create and continually improve search strategies.
ICSRs, aggregate reports, and any potential safety-related data should all be considered when developing search strategies. To reduce the chance of missing pertinent ADR information, it is crucial to create and continually improve search strategies. Specifically, query terms must be carefully crafted to pull up the most relevant publications that address safety concerns.
To ensure safety-critical signals are not overlooked, the database must be comprehensive and adhere to minimum requirements.
Pharmacovigilance searchers typically use two or more databases, usually three or more, as having access to more databases improves their ability to find recalls and ensures thorough coverage.
Use a search strategy that balances precision and accuracy demands. Local regulatory organizations advise local literature searches in regionally recognized databases.
 Overview
Overview
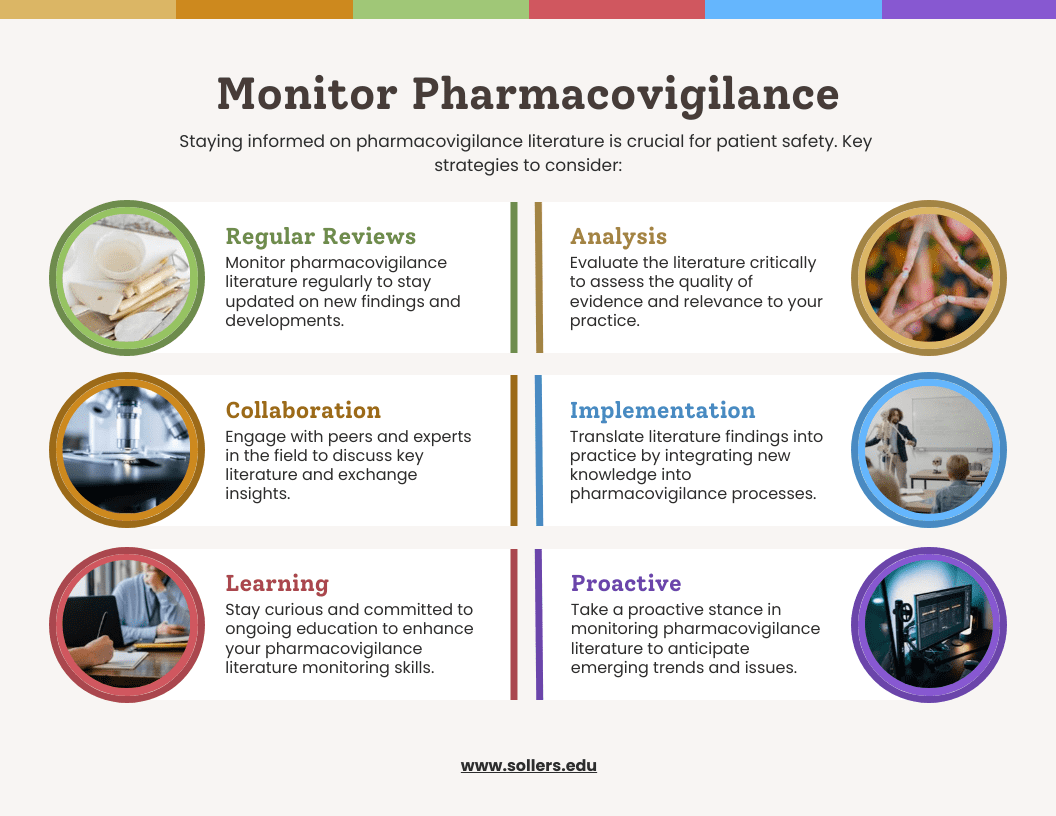
A significant amount of literature monitoring is involved in pharmacovigilance. It may be difficult, but developing a sound search strategy is crucial. Adverse event-related safety information will never be overlooked thanks to a professional with the necessary abilities, knowledge, and training. It is essential to create and maintain search strategies, gather suggestions from various stakeholders, and create approved and practical strategies for the task at hand. Establishing an elaborate process to handle and manage duplicate articles is essential. Review search tactics frequently and make sure the documentation is solid to ensure the best results. To determine whether the MAH’s literature monitoring systems adhere to high standards, consider the following factors:
- There is a need for a drug safety specialist with literature review knowledge.
- Conduct risk analyses to ensure the search criteria are reliable and pertinent to the literature search purpose.
- Perform a literature search and assess the literature findings by regional requirements (both global and local).
- To improve results, the search phrase is analyzed and updated annually.