The complexity and cost of clinical trials have increased dramatically in recent years.With rapid advancements in Clinical trials, the methodologies used to support vital clinical trials are necessary to protect patient safety. Efficient monitoring is becoming critical to protect the well-being of trial participants and to maintain the integrity of final results; Risk-Based Monitoring is now generally accepted that the process for clinical trial monitoring needs to change. As the inclination towards using Risk-Based Monitoring increases there still remains an inertia to opt for this method.
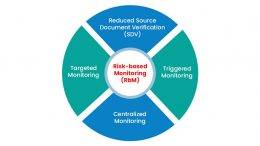
The importance of Risk-Based Monitoring:
Taking a risk-based approach to study quality and the monitoring of any clinical investigation has a simplistic focus:
- Recognize potential threats
- Create a plan to monitor those activities
- Improve monitoring methodology as needed
Centralized Monitoring:
A combined approach based on the recognized risk at each study site.
Remote Monitoring:
Utilization of low-cost clinical resources to perform monitoring activities that do not need onsite visits.
Reduced Monitoring:
SDV approach concentrates on patient visits, critical data, and selected patients, depending on the trial’s risk-benefit profile.
Triggered Monitoring:
Depends on predefined trigger points such as patient enrollment rate and reported Serious Adverse Events (SAE).
Let’s discuss the few essential needs for this:
Data Agnostic Integration:
In the ever-changing Clinical trial landscape, patient trends, and data requirements, RBM needs a technology platform to future proof against the challenges. Acquiring an agnostic integration platform can help a services business remain agile.
Risk-based monitoring(RBM)marks a paradigm shift from analyzing each piece of data from a trial to a more engrossed analysis that happens in real-time. Agnostic integration is a platform that can interface with any operating system and database, preventing incompatibility, data corruption, and vendor lock-in. An agnostic approach is to simplify integration between critical systems of record and other apps that help the service’s business lifecycle.
User Friendly Interface:
A clear dashboard provides an edible format for the users to spot difficulties early and is significant. Clear and easy-to-use data visualization requires to be combined with quick access to the data below to let study teams examine data in detail down to the levels of performance by site or country and create bespoke reports. Make convinced the platform you choose uses clear and steady navigation routes that are recognizable to users.
INTELLIGENT MONITORING
Part of the power of RBM is enabling sponsors and CROs to move to more intelligent monitoring methods that can direct resources to monitor the sites or subjects who are at the most significant risk. Ensure the technology platform you select supports intelligent monitoring with real-time visualization and flags of trial-level issues like recruitment delays or protocol deviations. Early oversight of risk empowers you to quickly identify problems and roll out prompt interventions, like protocol amendments.
When sponsors and CROs leverage available technologies and develop a risk-based monitoring plan upfront, it leads to less data collection for site personnel and less on-site data monitoring by CRAs. Clinical trials without efficient data collection technologies will continue to rely on the sites to scan pages and pages of documents so that CRAs can monitor remotely. This is not the intent of risk-based monitoring. For risk-based monitoring to work for sites, the trial must be designed to collect and analyze the data more reflectively and efficiently.
Risk-based monitoring is taking over as it promises greater accuracy, lower prices, in-depth analysis, and timely results. The Short-term Program from Sollers in Risk-based Monitoring is an added advantage.