2020 gave rise to the most devastating epidemic in modern history, but it also provoked amazing breakthroughs across Clinical research and Pharmacovigilance stewardship. Rising incidences of ADR during COVID vaccine have elevated the importance of Pharmacovigilance in the market.
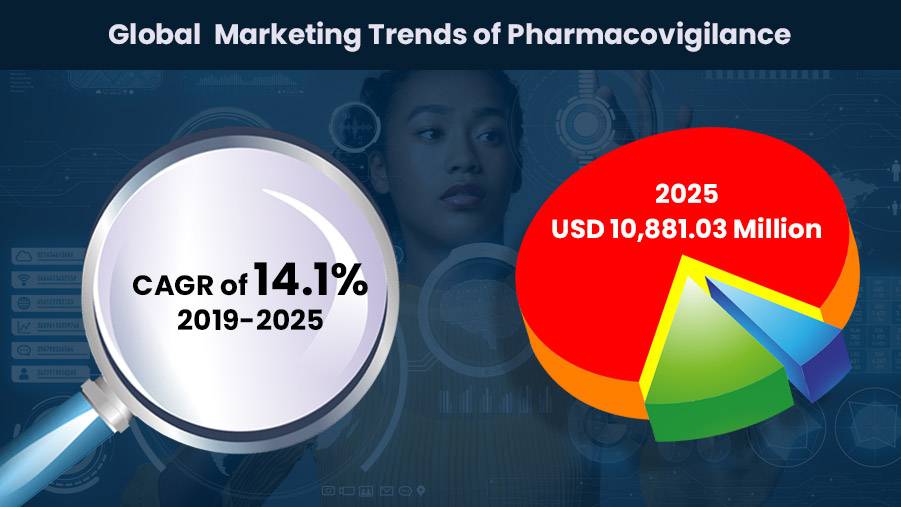
As per the reports, from 2020-2027, the market is estimated to grow with a 7.6% CAGR, and the global market is expected to grow nearly triple to $12 billion by 2027 with the surge in the application for electronic health record mining, intensified drug report monitoring, cohort event monitoring, spontaneous reporting.
There has been a steeping demand for pharmacovigilance services because of the developing predominance of chronic diseases, respiratory disorders, cancer symptoms, and rising drug consumption.In addition to this, a vast rate of adverse drug reactions (ADRs) have added a substantial strain on the healthcare sector, pointing to the augmented demand for Pharmacovigilance.
The increasing trend of outsourcing PV services to CROs and BPOs has derived from an effective drug regulation system. These outsourcing substances contribute to pharmacovigilance services with high regulatory compliance, improved productivity, and more remarkable strategic outcomes, thus expanding the global PV market.
The critical factor for expanding the pharmacovigilance market is the governments’ strict drug safety policies and regulations. Also, around 5% of total hospitalizations are due to those adverse reactions. Therefore, the scope of pharmacovigilance is cumbersome as countries have to produce solutions to evade these adverse drug reactions.
Intense post-market monitoring mechanisms set up by various government regulatory agencies increasingly focus on the safety and efficacy of pharmaceutical products after they are launched in the market.. So, there will be no shortage of jobs in this sector. Candidates will obtain many job openings in pharmaceutical companies along with a few positions in the public sector.
To make a more glorious future in Pharmacovigilance, enroll in Pharmacovigilance Courses to get the best training from industry experts. Sollers offers certification and a Master’s program in Drug Safety and Pharmacovigilance along with Adverse Drug Reaction programs.



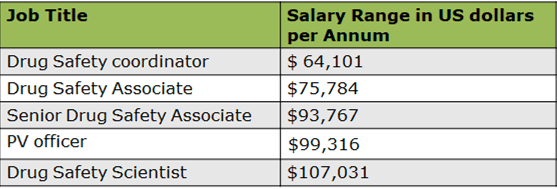
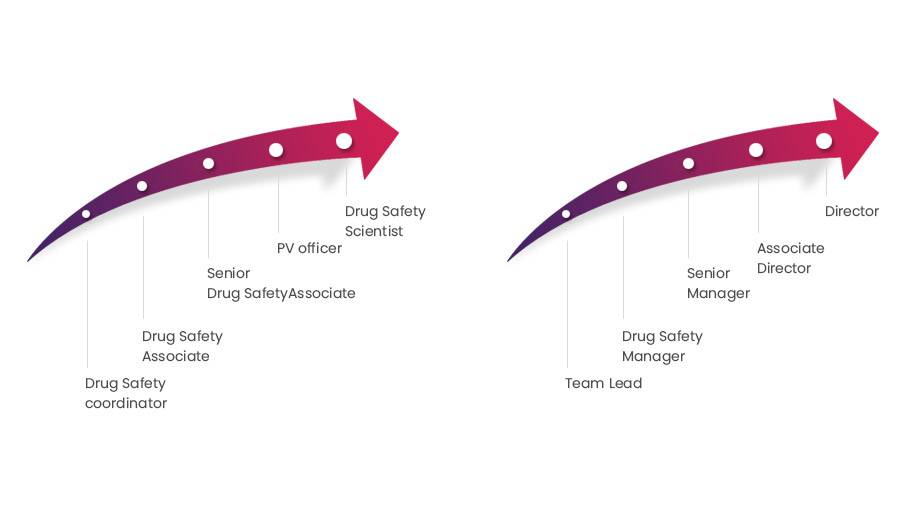 Drug Safety Coordinator:
Drug Safety Coordinator: