Life sciences organizations confront new difficulties in Clinical Trials, Drug Safety etc., and possibilities every year, but 2021 will be the year when they are front and center more than ever. According to the US Bureau of Labor Statistics, professions in the life, physical, and social sciences will expand by 7% by 2028. It will be quicker than the national average for all other vocations. Within the following decade, this would imply the creation of around 97400 new employment.
The COVID-19 epidemic has also shifted the job landscape by emphasizing the importance of the biological sciences industry.
Industry expansion is vital. Because of the pandemic’s need, there is a fierce and considerably more demand for talent than ever before. Employers have faced issues as a result of a rising supply and demand mismatch for trained individuals. Candidates with experience in areas such as healthcare, biotechnology, and pharmaceuticals will be in great demand. Companies would have to undergo a more extended recruitment procedure to fill life sciences, especially in specialist areas.
The need for technological skills is growing. With the convergence of technology and life sciences, there has been a greater emphasis on recruiting personnel with the skills to offer tech-enabled solutions. Biotech, data analysis, and digital product management will be in high demand in 2021 and beyond. Companies will remain ahead of the curve in a continuously changing industry.
Remote positions are becoming more prevalent. The pandemic has caused a shift in the rise of small labor in a variety of industries. Many conventional jobs in the life sciences industry have gone online and will continue to be distant in the future. The need for remote positions for healthcare practitioners such as physicians, nurses, and other clinicians has increased as telehealth services have been more widely adopted.
Biopharma and life sciences businesses are now competing with technology companies for specialized sector personnel, such as computational biologists and bioinformaticians, in addition to seeking the same talent pool as other industrial sectors for general digital skills.
Are you prepared to take chances on applicants with less experience but a desire for innovation?
That is very dependent on the function. In some instances, especially in specialized areas, you cannot afford to take the risk or devote the time to train individuals up. In other cases, hiring for potential and enthusiasm makes excellent sense.
Sollers provides certificate programs in various life science fields such as Clinical Research, Clinical Data Science and Drug Safety & Pharmacovigilance.











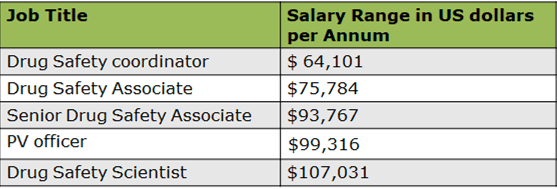
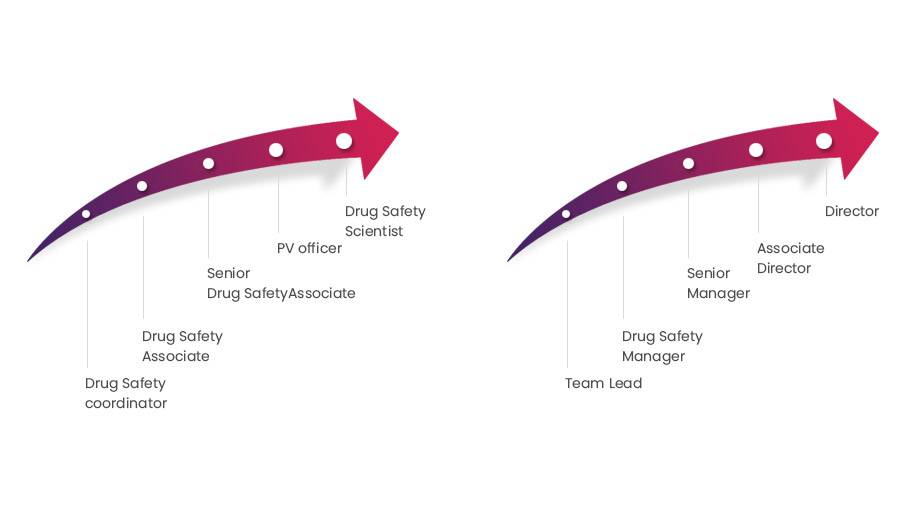 Drug Safety Coordinator:
Drug Safety Coordinator: