When COVID-19 shocked the world in 2020, the importance of drug safety jobs increased. As countries looked for the best way to combat the pandemic, pharmacovigilance also became a hot topic.
To determine the best outcomes for COVID patients while attempting to stop the virus in its tracks, the pressure was put on the life sciences market and the industry. Drug interactions and their effects are evaluated, monitored, and discovered in the healthcare system.
COVID-19 has been devastatingly impacting global health, but the pandemic and its demands have forced the pharmaceutical and pharmaceutical care industries to innovate and develop new methods that can collect and use more reliable and effective data about the safety of drugs.
The urgent task of gathering and analyzing data from pandemic clinical trials as well as post-marketing settings was suddenly given to pharmacovigilance teams all over the world. Pharmaceutical companies conducted research and innovation at this time to monitor vaccine efficacy and safety.
A growing pharmaceutical industry and increased global drug demand are driving up demand for pharmacovigilance experts.
Many of these innovative techniques improved pharmacovigilance and are now employed to keep track of both newly released medicines and those that have been on the market for some time. Better patient outcomes were the outcome.
But what exactly are these changes?
A better way to respond to change before the pandemic, the pharmacovigilance sector was resistant to change. However, the industry had to quickly adapt and think differently due to the urgent need for swift action.
The COVID-19 crisis forced teams in the life sciences to re-evaluate their methods and showed them that change can be a good thing.
The use of automation and AI tools are being expanded.
To meet the needs of the healthcare market while ensuring worker safety, many life sciences industries have evaluated and implemented cutting-edge technologies and tools.
The standards of drug safety procedures have continued to rise, thanks in large part to AI, which at this point became a crutch. The use of AI-enabled chatbots is now being extended to automate more administrative tasks and collect data where human error is unlikely to occur.
By automating time-consuming intake tasks, this tool allows healthcare professionals to concentrate on the tasks that are most relevant to patients.
Aside from AI, PV has been altered by:
- increased drug testing
- accelerated drug development
- Effective management and quality control
- accurate market analysis and forecasting
- Estimating product costs
Future developments are anticipated to meet the demand for personalized medicines and therapies as AI’s application to drug safety continues to grow.
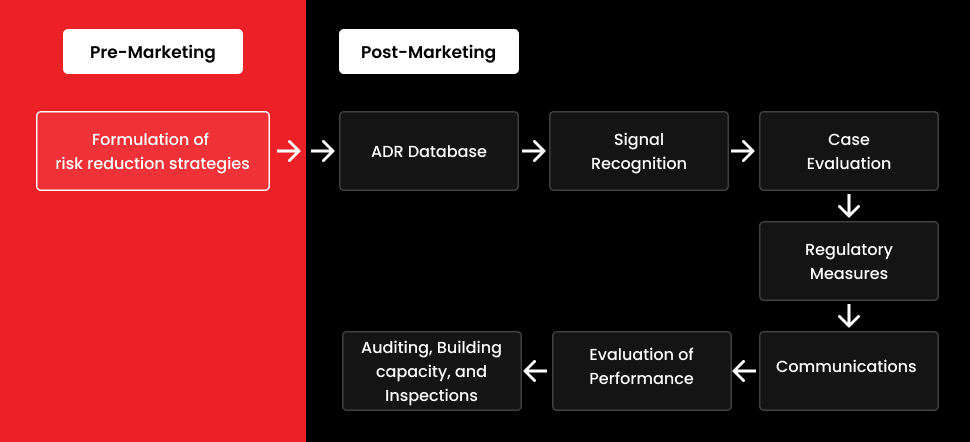
An updated era for tracking adverse reactions
Before the pandemic, adverse event reporting systems (AERS) were in use. These systems, which are a component of AI technology, became even more important in the effective monitoring of drug safety and public health when the pandemic occurred. To stop misinformation from leading to unnecessary hype and dangerous trends, diligent reporting was required.
The health industry is now more transparent because of this new era of adverse event reporting, giving patients clearer information about medications and treatments as well as their effects.
Sollers college is driven to produce results and is enthusiastic about the development of the health and life sciences industry.
Our top priority is to find the best candidates for the best PV roles so that the industry can advance. Students can continuously enhance this process to the values and tenacity of Sollers College.
For career advancement, look through our extensive list of pharmacovigilance positions. Do you wish to learn more? If you need assistance, please get in touch with us.