- Pharmacovigilance is the science of recognizing, assessing, comprehending, and preventing hazardous drug reactions.
- The main objectives of pharmacovigilance are identifying and assessing previously reported adverse drug reactions; assessing previously reported adverse drug reactions, and lowering mortality and morbidity associated with adverse events.
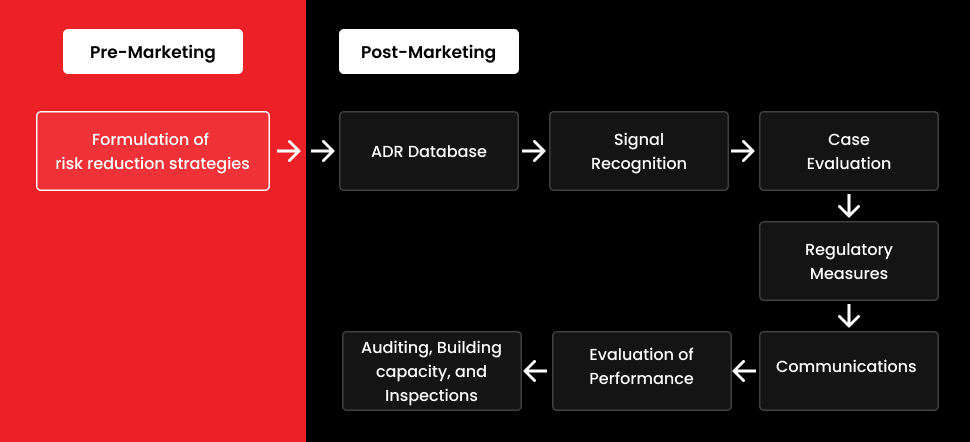
- PV, also known as post-marketing surveillance, is mostly done throughout the drug development phase.
- The most crucial part of pharmacovigilance is signal identification and evaluation.
- A signal, according to the WHO, is reported information on a potential causal association between an adverse event and medicine, of which the association is undetermined. Frequently, a signal is represented by a small set of reports.
- Signal identification and evaluation are crucial and intricate procedures. As a result, qualitative signal detection and assessment techniques utilized in pharmacovigilance.
An Analysis of Signals
The pharmaceutical industry and regulators are all very interested in the early detection of safety information as soon as feasible. Both qualitative and quantitative components make up signals.
Different approaches for detection are required for different categories of adverse events. Early signal detection is the main purpose of pharmacovigilance. However, procedures for reporting spontaneous events have been created and are now utilized globally.
Case-control, cohort, and spontaneous reporting are only a few of the sources that produce safety signals.
Automatic Reporting System
- Most of the current pharmacovigilance relies on a spontaneous reporting mechanism. The spontaneous reporting system often includes case reports and case series. Early detection of signals from new, uncommon, and severe ADRs is the primary purpose of SRS.
- A medically qualified person reports an incident voluntarily to a drug information center, where the reports are analyzed. Spontaneous reports are used to keep track of the underreporting of adverse medication responses and quality deviations. Underreporting is the main reason for the public’s lack of understanding among health professionals and the public.
- Another issue in this system is selective reporting, which can create the perception of a risk when there isn’t truly one. Therefore, even though spontaneous reporting is inexpensive, it is not the ideal solution for postmarketing drug surveillance.
- Nevertheless, we cannot dispute the fact that spontaneous reporting was and continues to be the primary method of identifying early drug safety signals. As evidence of SRS’s effectiveness in identifying fresh safety signals, most pharmaceutical goods are pulled off the market on its premise.
Recurrent Safety Update Report
- The PSUR can be a valuable resource to find novelty signals. The purpose of a PSUR is to inform the competent authorities at specific intervals after permission of an update on the global safety experience of a medical product.
- PSURs must be submitted for all registered products, no matter how the product is marketed. One report may be used to cover all items authorized by one marketing authorization holder that contains the same active ingredient.
Trigger Tools Are Used to Produce Signals
- Healthcare professionals are looking for an accurate and trustworthy technique for measuring and identifying adverse drug reactions in hospitalized patients.
- The clinical pharmacist monitors the efficiency of drugs using electronic systems and is responsible for identifying early adverse drug reactions and other drug-related issues.
Examination of Signals
- Multiple criteria are used to assess signals. Before considering a report of a brand-new adverse drug reaction, high-quality report facts must be there.
- Numerous tools are used to create high-quality data, including various applications and techniques.
- Additionally, a few studies have been published to demonstrate the relationship between cause and effect, but regrettably, there is no widely accepted method for identifying the cause of ADRs.
Quality Control
- Signals having insufficient information might render determining an event’s cause unfeasible. The information on patients and medications is the essential foundation for the subjective evaluation of the quality of the reports.
The strength of the adverse event
- The incident’s description and the data provided in the pertinent section of the ADR forms are used to determine how serious the event is.
- Adverse occurrences are considered serious if they were fatal, life-threatening, resulted in significant impairment or incapacitation, or required extended hospitalization.
System for Reporting Adverse Events
- The FDA’s Adverse Event Reporting System is a database that contains information on reports of drug mistakes and adverse events. The FDA’s post-marketing safety surveillance program for pharmaceutical and therapeutic biologic products is supported by the database.
- Adverse events and medication errors are classified by Med DRA nomenclature.
- The AERS can be used by the FDA to perform duties like looking for recent safety concerns that might be related to commercially available products and evaluating a manufacturer’s compliance with reporting requirements.
The Argus Safety Database
- One of the most important components of the pharmacovigilance software system is the Argus Safety 3.0.1 database. Employers can use the digital database to support pharmacovigilance and other relevant operations while ensuring compliance with international laws.
- It provides a pharmacovigilance business process that occurs during the drug’s pre-and post-marketing phases as a full pharmaceutical software solution. The Argus database is housed in an ISO-9001 accredited data center that complies with the safety regulations set forth by the FDA regulations.
- Oracle Argus Safety products that are related to Oracle Argus Safety include Oracle Argus Insight, Oracle Argus Perceptive, Oracle Argus Affiliate, Oracle Argus Dossier, Oracle Argus Interchange, Oracle Argus Reconciliation, and Oracle Argus Unblinding.
Recent Advances in Methodologies
- Risk management plans have recently been established in post-marketing surveillance to systematically characterize, prevent, or limit hazards associated with pharmaceutical products, including the evaluation of the intervention’s efficacy.
- The benefits-risks of the medicine throughout the post-authorization phase can be better comprehended with the aid of these RMPs.
- To effectively identify the warning signs of adverse events, health professionals must present accurate information in their adverse event reports. The quality of adverse event reports is improving as more reports are made online.
- Another crucial breakthrough is patients’ taking part in pharmacovigilance. Patients can now report ADRs to the spontaneous reporting system in many nations. Data can be collected and analyzed quickly with this kind of automation.
Further Outlooks
- Academics must create fresh approaches that can improve the current system to further demonstrate pharmacovigilance’s scientific validity. Active observation is required to learn about the drug’s safety at an early stage.
- One must keep the significance of being able to obtain information promptly in mind when creating new techniques for active post-marketing surveillance. In most cases, the techniques, and the results conflict. Therefore, it’s critical to provide techniques for answering this kind of query.
- Patients’ roles are progressively evolving. The patient is now well-informed about his illness and eager to take an active role in his care. Therefore, in the future, pharmacovigilance must focus on this group as a key source of information.
- Future pharmacovigilance must be capable of quickly recognizing novel safety signals. If this is successful, the patient’s faith in medications will return.
The scope of the research
- The most crucial part of pharmacovigilance is accurate signal identification and assessment. Signal detection is accomplished using a variety of techniques. Pharmacovigilance signals come from a variety of sources.
- PV may not be dependent on a single technique but rather on a coordinated set of actions. Through effective training and retraining of the staff involved in the pharmacovigilance activity, the quality of the reports can be enhanced.
- No single causality assessment technique is accepted by everyone. Therefore, the current desire is for a single effective strategy that is accepted by everyone.
Sollers College will help you bridge the gap between these lucrative jobs and the skills required by prospective candidates. A career in pharmacovigilance affords you the chance to make a difference in people’s lives due to the current increase in the need for pharmaceutical specialists.