In the ever-evolving world of pharmacovigilance (PV), aggregate reporting stands as a cornerstone of drug safety. As the pharmaceutical industry expands globally and regulatory requirements tighten, professionals trained in aggregate reporting are increasingly in demand. But what exactly is aggregate reporting, and why is it critical for anyone pursuing a career in drug safety?
Understanding Aggregate Reporting in Pharmacovigilance
Aggregate reports are comprehensive documents that summarize the safety profile of a drug over a defined period, typically combining data from multiple sources such as spontaneous reports, clinical trials, and published literature. These reports help regulators and pharmaceutical companies detect trends, and emerging safety issues, and assess risk-benefit profiles.
The most common types of aggregate safety reports include:
Periodic Benefit-Risk Evaluation Reports (PBRERs) – An internationally standardized format that has largely replaced the traditional PSUR under the ICH E2C(R2) guideline.
Periodic Safety Update Reports (PSURs) – Still used in some regions, but often referred to as PBRERs in the current regulatory landscape.
Development Safety Update Reports (DSURs) – These are required during the clinical development phase of a drug to monitor safety in ongoing clinical trials.
These reports are required by health authorities like the FDA (U.S.), EMA (Europe), and other regulatory bodies worldwide.
Why Is Aggregate Reporting So Important?
Aggregate reporting ensures that drug manufacturers maintain transparency and accountability throughout the lifecycle of a pharmaceutical product. It helps prevent adverse events, protects patient safety, and maintains compliance with global regulations.
Here’s why you must pay attention to aggregate reporting if you are entering the field of pharmacovigilance:
- Career Relevance: Most global pharmacovigilance roles, from safety officers to regulatory specialists, require a strong grasp of aggregate reporting.
- Global Standardization: Aggregate reporting is recognized and mandated by international regulatory authorities, opening doors to global career opportunities.
- Real-World Impact: Your work directly contributes to safer medication use worldwide.
Current Industry Trends & Market Insights
The demand for skilled pharmacovigilance professionals has been rising steadily due to increased drug development and stricter regulatory scrutiny.
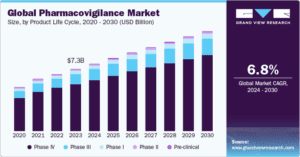
Global Market Projection: The pharmacovigilance market is expected to reach USD 13.9 billion by 2030, growing at a CAGR of 11.5% from 2023. (Source: Grand View Research)
Growth in PV Roles: Job postings related to pharmacovigilance and drug safety reporting have increased by over 25% in the last 2 years on platforms like LinkedIn and Indeed.
Average Salary: Entry-level roles in aggregate reporting start at $60,000/year, while experienced professionals can earn up to $120,000/year, depending on location and qualifications. (Source: Glassdoor)
Global Reach: With regulators like the European Medicines Agency (EMA) and U.S. FDA mandating standardized safety reports, aggregate reporting professionals are now integral to global pharmacovigilance operations.
Get Trained and Future-Ready
At Sollers College, our specialized programs in Drug Safety and Pharmacovigilance include comprehensive training in aggregate reporting. You’ll gain practical exposure through real-world case studies, tools, and hands-on reporting simulations.
Whether you have a background in life sciences, nursing, pharmacy, or medicine, learning aggregate reporting equips you with in-demand skills that pharma companies are actively seeking.