Master of Science in Clinical Trial Management
Work with the latest industry tools and learn how to apply them in the domain of clinical trials.
OVERVIEW
 Why Clinical Trial Management?
Why Clinical Trial Management?
The Master of Science in Clinical Trial Management is a highly-selective program for students with a strong biological science or medical background. area that includes medicine, nursing, dentistry, pharmacy, microbiology, biotechnology, biochemistry, and similar areas. Students with a background of information technology, bio statistics, and other general commerce and finance backgrounds can also take this as an additional specialty option after some prerequisite training.
 In this Course
In this Course
Our Graduate/Master’s program includes 20+ weeks of Internship to provide rigorous hands-on experience in Clinical Trial Management System (CTMS), Electronic Trial Master file (ETMF),Argus safety database, adopted by the pharmaceutical industry today, and in SAS (Statistical Analysis System) through real-time Case Scenarios.
Clinical SAS programming and Risk Based Monitoring is one of the important electives in the program. In the Clinical SAS module, the Base is covered along with Advance SAS Programming, including CDISC, SDTM, and ADAM.
The Clinical data science track will deal with basics of statistics, introduction to sampling techniques, probabilities and statistical tests for discrete and continuous random variables including R, Data Visualization and SQL.
Students can apply to various roles Clinical Research associate,Clinical research coordinator, Clinical research assistant,Clinical data analyst, clinical SAS programmer, Clinical data Scientist, Data Entry specialist
HIGHLIGHTS
![]() Proficient Instructors: Experts with 15+ years of industrial experience guiding students and providing real-time examples and scenarios.
Proficient Instructors: Experts with 15+ years of industrial experience guiding students and providing real-time examples and scenarios.
![]() Software and Tools: Get in-depth knowledge of CTMS, eTMF and SAS tools based on elective selection.
Software and Tools: Get in-depth knowledge of CTMS, eTMF and SAS tools based on elective selection.
![]() Internship Program: Internships in Clinical Trial Management PV based on elective selection.
Internship Program: Internships in Clinical Trial Management PV based on elective selection.
![]() Curriculum: Job-oriented, industry-based curriculum aligned with the current scenario with full-fledged access to program content, batch recordings, and tools after one year of program completion.
Curriculum: Job-oriented, industry-based curriculum aligned with the current scenario with full-fledged access to program content, batch recordings, and tools after one year of program completion.
![]() Certification: Two certificates – a course completion certificate and internship completion.
Certification: Two certificates – a course completion certificate and internship completion.
![]() Career Guidance: Our career service advisors provide guidance for the resume and interview preparation.
Career Guidance: Our career service advisors provide guidance for the resume and interview preparation.
![]() References:References for the jobs as a Clinical ResearchIntern.
References:References for the jobs as a Clinical ResearchIntern.


LEARNING OUTCOMES
- Understand ethics, federal regulations, good clinical practice (GCP), and how the FDA regulates the drug commercialization process.
- Understand HIPAA law, the protected health information, and its implications.
- Explain the concept of clinical trial management (CTM) by understanding the fundamental concepts of study planning, study setup, conduct, and closeout.
- Students can choose the Argus safety database as one of their specialties in their semester.
- Describe data manipulation techniques using SAS DATA and procedure steps to access, transform, and summarize SAS data sets.
Syllabus
Register now, to get the complete syllabus.
INTERNSHIP
Sollers offers an internship as part of the Master of Science in Clinical Trial Management program that helps you gain practical experience on key concepts taught during the course.
Upon successful completion of the program, a certain level of skill and expertise is expected of students to get placed with our industry-leading corporate partners.
Instructors
Our instructors are not just highly experienced in the industry, they give you the personal attention you need and guide you every step of the way.
Course Duration
 Duration
Duration
2 Years
For information regarding fee and/or reserving your spot, contact our Admissions Team.
Credit transfers applicable for alumni

Career Guidance
After the completion of program, we assist our students with interview coaching, resume building sessions, conduct mock interviews, job readiness training and make them competent to venture into the corporate world.

Student Testimonials
FAQs
According to Marketwatch, a Bachelor’s Degree holder will earn over 26% more money than an Associate’s degree holder with similar experience. Higher education has never been more important, in today’s fast-paced, technology-driven, highly competitive world. While you don’t have to invest in 4-5 full years in education if you don’t want to, getting an accelerated Bachelor’s degree like this one will definitely allow you to take few special courses and give you a head-start to set you on a right career track.
The high growth of the industry and career prospects in clinical research are already stated in the introduction above. There is practically no other comparable bachelor’s degree program in clinical research in the market today: that’s right, this is the first-of-its-kind accelerated bachelor’s degree to help you start in the lucrative career of clinical research in almost half the time and investment you’d have to put in a traditional masters or bachelors degree. Once you sign up, you get the training as well as relevant experience through our internship, to show for your first job – something that is extremely hard to get these days. Our career services team prepares you through career counselling, resume reviews, mock interviews and soft skills training. Not convinced yet? Contact us to discuss how we can specifically help you and how exactly can this program add value to your career.
This is an accelerated bachelor’s program. This associate to bachelor’s degree program is for total of 60 credits, and can be completed in a year and a half to two years.
Yes, we can allow credit transfer upon certain conditions; however, each request will be evaluated case-by-case basis. Please contact us on [email protected] to learn more.
There are no prerequisites, except that you need an Associate degree, preferably in any of the life sciences, biological sciences or relevant areas.
One of the key highlights of this program is the internship and hands-on, practical projects – all of which give you the relevant experience to not only understand and talk intelligently about clinical research, but help you get that first job in the industry. You will gain extensive practical sessions in Clinical Trial Management systems, Oracle Clinical – most widely used data management system in pharmaceutical industry – and SAS (Statistical Analysis System) as well as use electronic Trial Management File.
Industry Facts
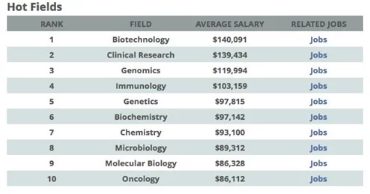 Given the fact that people are going to fall sick, and pharmaceutical industries will have to continue producing life-saving drugs, clinical research is a recession-proof, one of the hottest careers today. Also, as personalised medicine becomes more common, clinical trials will increasingly be needed to make sure drugs and devices work properly.
Given the fact that people are going to fall sick, and pharmaceutical industries will have to continue producing life-saving drugs, clinical research is a recession-proof, one of the hottest careers today. Also, as personalised medicine becomes more common, clinical trials will increasingly be needed to make sure drugs and devices work properly.
The global clinical trials market was valued at US$40 billion in 2016, and is expected to reach US$65.2 billion by 2025, according to a recent grand view research report. North America dominated the overall market due to big outsourcing firms and increase in R&D, according to the same report. According to the Scientist’s 2015 Life Sciences Salary report, Clinical Research is one of the top hot fields and ranks second in salary.

 In this Course
In this Course



