Advanced Clinical Research
Learn to conduct clinical research, collaborate with clinical scientists and enhance your skills in patient-oriented research.
OVERVIEW
 Why Clinical Trial Management?
Why Clinical Trial Management?
This course provides training for students to learn how the Clinical Trial Management process works, developing complete Regulatory activities involved in Creating Documents and in Planning, Organizing, Monitoring, Recording, Analysis and Reporting of Clinical Trials. The program provides additional opportunities to learn about recent advancements as well as specializations in Risk-based Monitoring concepts.
 In This Course
In This Course
Study Startup: Understand the phases of the Clinical Trial, ICH-GCP guidelines, Study Feasibility, and Site Selection. Study Conduct: Learn about Site Monitoring Visits, which include Remote and Central Risk-Based Monitoring. Study Closeout: Learn about Site Close-Out Visit including both On-Site and Remote activities.
 The Sollers Advantage
The Sollers Advantage
Work on real-time Case Scenarios, Clinical Tasks, and other Site Management activities taught by industry experts.
LEARNING OUTCOMES
- Understand and implement Site selection, Site initiation, Site monitoring, and various other Site activities Activities
- Students will assign sites, Site staff, IRB, and labs to the Pre-designed studies in the CTMS as a CRA
- Students work on various Documents related to Site Visits as per the regulatory bodies requirement
- Students will work on the Site binder and trial binder in the eTMF System
- Students will be working with documentation related to local and central IRB (Regulatory board)

Syllabus
Register now, to get the complete syllabus.
INSTRUCTORS
Our instructors are not just highly experienced in the industry, they give you the personal attention you need and guide you every step of the way.
Course Duration
 15 weeks (20+ hours each week)
15 weeks (20+ hours each week)
3 Sessions /week
 Engagement
Engagement
150 hours
For information regarding fee and/or reserving your spot, contact our Admissions Team.
Credit transfers applicable for alumni

Career Guidance
After the completion of program, we assist our students with interview coaching, resume building sessions, conduct mock interviews, job readiness training and make them competent to venture into the corporate world.
We provide exclusive one-on-one sessions with our industry-based career advisors who provide guidance right from resume feedback, assisting with interview Q&As, and helping with job preparations.

Student Testimonials
Campus Visit
FAQs
This is a 300 Hour program
Graduates from science, nursing, pharmacy, medical, dental, and computer background can take this program
Basic knowledge to work with Computers, quick learning aptitude to get trained in newer software application tools, company SOP, Medical terminology, Clinical SAS, willing and ready to travel.
Understanding of ICH/GCP clinical trial paradigm, Roles and responsibilities of various functionaries of Clinical trial process.
Detailed training of Clinical Trial Manager, Monitor, Coordinator, Data analyst functions
Be proficient to work on EDC based Risk Based Monitoring along with user friendly knowledge of relevant tools like CTMS, eTMF, Clinical SAS.
Excellent and versatile senior faculty from Clinical trial industry.
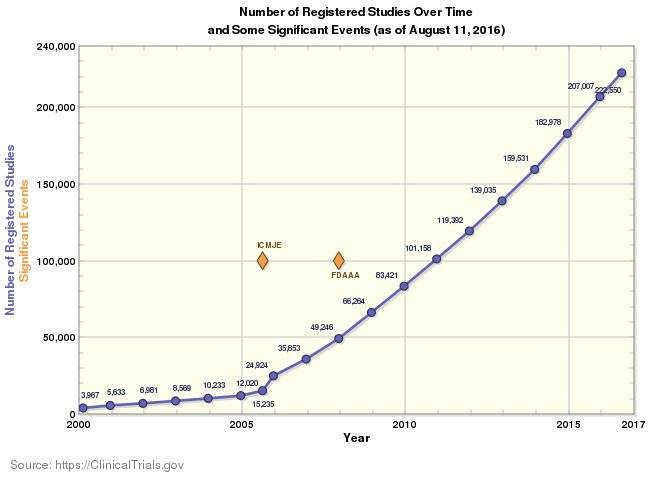
Clinical Trials are ever expanding area for drug development process.
More than 20,000 Trials were added during 2016 with annual increments of at least 6% per annum.
ClinicalTrials.gov reports a total of more than 250,000 Trials
Industry Facts
Clinical Research has proven to be exceedingly more necessary by health organizations and pharmaceutical companies in today’s modern age. This trend can be summarized by two realities: higher rates of chronic diseases and a fast-growing global population. These two elements combined, create a need for the technological advancement throughout the industry; methods and techniques that can be developed quickly to help treat the growing medical issues of today and of the future (Yau, Promising Futures for Scientific and Clinical Research Professionals, 2014).
Clinical Research has just been ranked as one of the hottest jobs with the 2nd highest salary in the life sciences sector in a BioSpace news article today. (READ MORE)
The highest paying life sciences jobs.
Importance of clinical trials. (READ MORE)
Growing demand for clinical trial studies justifying the demand for qualified clinical trial professionals.

 In This Course
In This Course The Sollers Advantage
The Sollers Advantage