May 12, 2025
Published by Sollers college at May 12, 2025
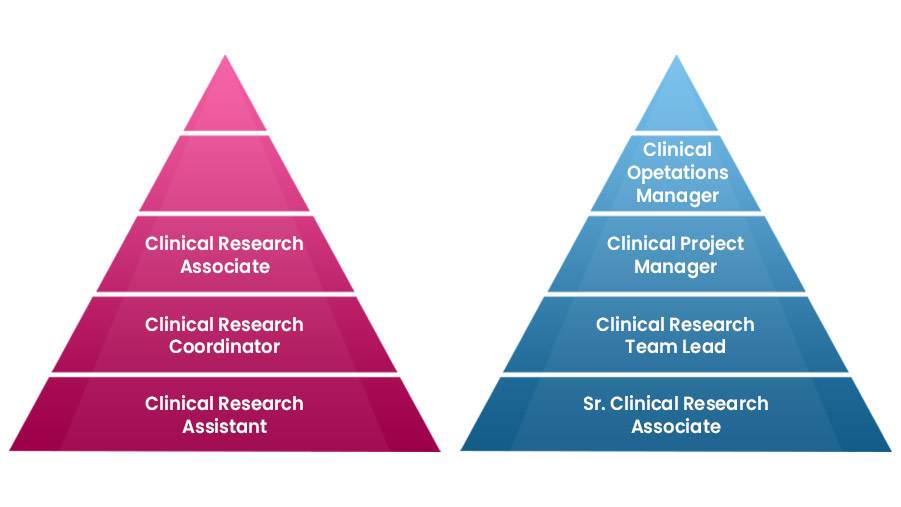
Introduction The U.S. healthcare and pharmaceutical industries are rapidly expanding, particularly in clinical research. This surge presents an incredible opportunity for International Medical […]
April 28, 2025
Published by Sollers college at April 28, 2025
Introduction The U.S. healthcare system is undergoing a data and innovation-driven transformation—and at the heart of this revolution is Clinical Trial Management (CTM). […]
April 7, 2025
Published by Sollers college at April 7, 2025
In today’s rapidly evolving healthcare ecosystem, the demand for professionals skilled in drug safety and pharmacovigilance is higher than ever. From biotech startups […]
February 10, 2025
Published by Sollers college at February 10, 2025
Pharmacovigilance relies heavily on medical coding, which is the methodical categorization and indexing of adverse drug reactions (ADRs) and other medical events. Medical […]
January 20, 2025
Published by Sollers college at January 20, 2025
Pharmacovigilance organizations are increasingly using automation technologies to optimize and expedite the medical coding process. Using machine learning (ML) and artificial intelligence (AI) […]
July 26, 2024
Published by organic@admin at July 26, 2024
It is critical to determine the safety and efficacy of various medical treatments, devices, and medications in the rapidly developing health industry. This […]
June 30, 2024
Published by organic@admin at June 30, 2024
New technologies and regulations are shaping the landscape of drug development and manufacturing leading to continuous evolution in pharmaceutical industry. Active Pharmaceutical Ingredients(APIs) […]
May 29, 2024
Published by Sollers college at May 29, 2024
Medical professionals are essential to drug testing, helping from the very beginning to the point of approval. Their participation guarantees clinical trials‘ efficacy, […]
May 16, 2024
Published by Sollers college at May 16, 2024
An autonomous clinical trial: what is it? The terms virtual, home, remote, and siteless are some of the terms used to characterize the […]
April 19, 2024
Published by Sollers college at April 19, 2024
With the advent of new treatments, adaptive design, and personalized medicine, clinical trials are more complex than ever. Sponsors aim for more agility […]
September 22, 2021
Published by Sollers college at September 22, 2021
Are you trying to shift towards a major career change path or simply looking for something new and distinct? a career in life […]
January 11, 2021
Published by Sollers college at January 11, 2021
Before the global pandemic struck, the world was turned on its head towards artificial intelligence (AI), especially the branch of AI known as […]
October 23, 2020
Published by Sollers college at October 23, 2020
With the advent of industrial advancement, the pharmaceutical sector has grown to its full power and envisioned increasing consistently in the coming years. […]
September 14, 2020
Published by Sollers college at September 14, 2020
Drug Safety and Pharmacovigilance radically transforming the future of the Pharmaceutical industry. The healthcare sector’s never-ending progress has resulted in modern medicines’ availability […]
July 1, 2019
Published by Sollers college at July 1, 2019
Statistical Analysis System, or SAS, has been the industry standard for data management and analytics of large volumes of data. It’s a very […]