
To gather information about adverse events and address patient safety, pharmacovigilance is essential. The AE case processing segment, a crucial component of PV, currently faces many difficulties.
Adverse event reporting is costly, time-consuming, and subject to human error, which could hurt patient safety. The industry will significantly benefit in the future from the promise of intelligent automation made possible by artificial intelligence and machine learning.
Artificial intelligence (AI) and machine learning (ML) have the potential to revolutionize the sector through automation technologies.
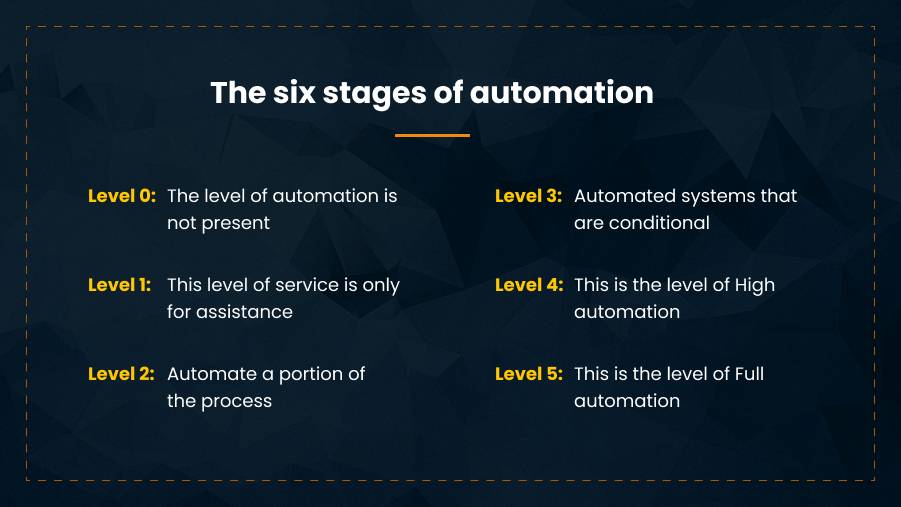
Level 0: The level of automation is not present
Stage zero, or manual case processing, excludes any automation support. The entire case intake and processing process are fully manual, and the case submission process and any decision-making that results from it are entirely handled by human users. Intervention systems may improve this performance, but the execution of the process remains schematic.
In terms of balancing compliance and cost, this may be all that is ever required for organizations with low volumes of adverse events. Several digital tools exist to automate steps in the process, which provide progression to the next stage.
Level 1: This level of service is only for assistance
Initially, autonomy can only help; human supervision is essential.
For many years, PV has included this level of automation to varying degrees. Auto-narratives and letter generation are two examples of how proven tools transform structured case data by manually selecting from pre-defined templates that are set up to match company-specific nuances. Instead of investing time and resources in manually creating these free text summaries, these examples show how auto-narratives and letter creation work.
Currently, most PV departments are automated with only minimal assistance.
Level 2: Automate a portion of the process
As automation advances, fewer scenarios call for manual intervention, though human users still monitor case processing and still intervene when necessary. In our analogy of an autonomous vehicle, automation enables the system to take over multiple tasks at once, such as steering, acceleration, and deceleration.
For instance, automating data extraction and identification from source documents speeds up case processing; doing this work in advance improves the ability to identify follow-ups and reduces the need for multiple copies.
Level 3: Automated systems that are conditional
Another situation where AI can do work-intensive tasks is bulk literature screening. Smart algorithms can parse more content in less time with better accuracy than PV professionals could by reading peer-reviewed literature and reports for AE signals over hundreds of hours.
Although there will be false positives requiring review, it is interesting to note that an automated PV system using natural language processing is more likely to find arbitrary mentions of ailments or products than a human reader. Alternatively, this can be done algorithmically, as it is when associated with null flavor values.
In either case, functionality reducing the need for human resource usage lowers the costs of pharmacovigilance, improves safety outcomes, and frees up PV professionals for more value-driven activities.
Level 4: This is the level that represents a high degree of automation
Due to its high level of automation, the system can handle each aspect of numerous case types. System quality and compliance are not always ensured by manual action, even when notified by human users.
At this point, the system can carry out all the operations necessary to receive and register a case report. This includes validation, duplication checks, and data entry from compatible systems. Medical evaluations are possible for a wide range of case types, and ML is increasingly enabling QC.
Following learned criteria, the triage function assigns cases a ranking for reporting purposes; a request for intervention is only made when a case deviates from the data’s assumptions. Depending on the health authority in question, the client risk profiles, and how well the case complies with other requirements, manual intervention may or may not be necessary when submitting to authorities or partner organizations. Depending on several factors, case closure and archiving can be automated.
Level 5: level of complete automation of the process
A PV system can execute autonomous case processes and submissions for all case types once it reaches full safety automation status. For any given case or report recipient, no manual intervention is necessary.
When the process is fully automated, AI can act to reduce patient risk if the system sends an intervention request, but no human responds appropriately. The actions are pre-set and automated for safety reasons, whether that means launching a national alert about a drug-related adverse event or putting a hold on dispensing a batch.
Positive effect on the economy
The life sciences industry gains from autonomous case processing in several ways. The traditional case processing workflow can be turned on its head, and labor-intensive and expensive processes can be automated, saving organizations time and money.
As a first step, companies need to assess their current situation and set precise goals in terms of PV automation. Risk tolerance should be considered when setting these goals. Even though there is no one-size-fits-all strategy, everyone should start small and introduce automation gradually as technology develops to the point where it can solve unimaginable problems.
Sollers partners with industry-leading employers in all fields.
Sollers prepares a comprehensive plan from start to finish based on a detailed analysis of your needs. Students will gain both the background and skills they need to succeed at an affordable price.
Get your career off to a successful start.



